
David Ducroq, Deputy Director
EQALM Barcelona 2016

Casa Milà

La Boqueria Market

Montjuic

La Sagrada Familia
Our lab manager, Samantha Jones and myself attended this important European meeting set in the vibrant city of Barcelona. The meeting is the annual forum for EQA providers to exchange ideas (sometimes argue) but generally just to meet up with like minded colleagues. Weqas presented posters on the traceability of glucose methods, analytical sensitivity of troponin assays and Weqas’ experience with the FOB/FIT programme (see hyperlinks below).-
- Traceability of Glucose Assays in the UK- Comparison with JCTLM listed Reference Method
- An EQA Scheme for qualitative FOB/FIT- a 2 year experience
- Analytical sensitivity of current Troponin assays
Early rise on day 1 for the working groups. Sam and I attended a lively discussion regarding frequency of distribution of EQA samples and the impact on analytical quality. A number of studies have already been undertaken by the Group to look at the evidence base. The studies to date suggests that the EQA schemes distributing samples on a more frequent basis result in better analytical quality, Weqas being a prime example.
The first day of the main conference opened with a session on Traceability and the change in focus of the next revision of ISO 17511 to include traceability to patient samples. The scope of this document will include measures taken to assure traceability in calibrators and patient samples. A questionnaire circulated by Eqalm looking at the Reference Measurement target values used in the various EQA schemes provided some interesting data. Of the returned data, only 10% of the EQA organisations use Reference Measurement values as their target value.
The evening social dinner event allowed an excellent opportunity for networking amongst the attendees. Unfortunately due to an unexpected lengthy thunderstorm, this meant we had to undertake a bus tour of Barcelona rather than the planned walk along the Ramblas pre conference dinner.
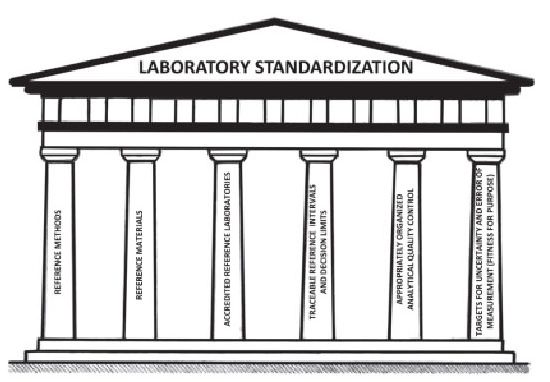 Day two started with a view of ”the perfect EQA” – is there another one ??
Day two started with a view of ”the perfect EQA” – is there another one ??
The requirements of ISO17043, traceability of the target value – the 6 pillars of traceability and 6-sigma principles for scoring of laboratories were covered.
An area of concern for most EQA providers (and often not communicated to participants) is the commutability of EQA material. Many issues can influence the commutability of both EQA and reference materials. The way in which the material is manipulated can affect the various measurands within the sample e.g. pH of the material may influence the isoforms of proteins and peptides in a sample. The best sample is the endogenous patient sample but EQA providers often have to compromise to ensure stability. An IFCC working group has been established to look at commutability issues.
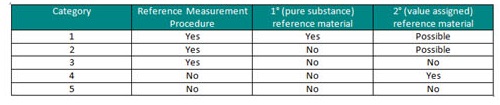
With the continued focus on traceability the work of the RELA surveys for monitoring the performance of Reference Laboratories was discussed. Where no reference measurement procedures are available demonstration of traceability is more difficult. In these situations , the manufacturer selected procedure may be used to define the kit targets (Cat 5), and Internationally agreed conventional calibrators (Cat 4). The hierarchy for the categories for a reference system are listed in ISO17511.
Categories for Reference Systems (ISO17511)
Other areas of discussion included Reagent lot recording to monitor Batch to batch variability. The more information participants provide to the EQA provider, the easier it is to identify trends.
Overall this was a great meeting, with lively discussions and an opportunity to catch up with colleagues. And next year in Ireland – what’s next on the horizon??
Last updated: 10/07/2020








